RUXOLITINIB VERSUS BEST AVAILABLE THERAPY IN PATIENTS WITH POLYCYTHEMIA VERA: 80-WEEK FOLLOW-UP FROM THE RESPONSE TRIAL
(Abstract release date: 05/21/15)
EHA Library. Kiladjian J. 06/13/15; 103108; S447

Prof. Jean-Jacques Kiladjian
Contributions
Contributions
Abstract
Abstract: S447
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A8
Background
RESPONSE, an ongoing, multicenter, open-label, phase 3 trial, compared the efficacy and safety of ruxolitinib (Rux) with best available therapy (BAT) in patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Primary analysis results — at 48 weeks from last patient first treatment (LPFT) — have been published (Vannucchi. NEJM 2015;372(5):426-435).
Aims
To perform a second preplanned analysis assessing the long-term efficacy and safety of Rux 80 weeks after LPFT.
Methods
Patients ≥18 years of age, who were resistant to or intolerant of hydroxyurea per modified ELN criteria, with splenomegaly, and phlebotomy requirement to control hematocrit (Hct) were eligible. Patients were randomized 1:1 to receive open-label Rux 10 mg BID or BAT; the latter was selected based on investigator’s choice. Patients randomized to BAT could cross over to Rux from week 32. The primary response was a composite of (1) achieving a ≥35% reduction from baseline in spleen volume by imaging at week 32 and (2) Hct control without phlebotomy through week 32 (defined as no phlebotomy eligibility between weeks 8 to 32 with no more than 1 phlebotomy eligibility from randomization to week 8). Durability of the primary response, Hct control, spleen volume reduction, complete hematologic remission (CHR), as well as long-term safety, were evaluated.
Results
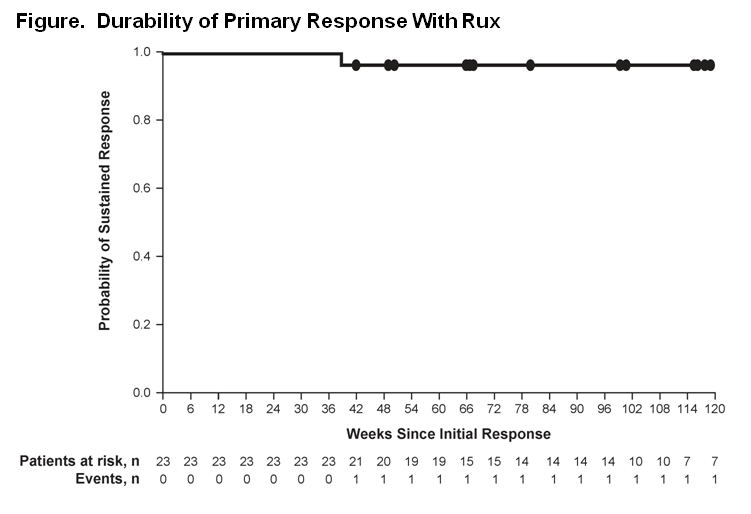
Overall, 222 patients were randomized (Rux, 110; BAT, 112). At the week 48 analysis, 93 (84.5%) patients randomized to Rux (Rux arm) were still receiving treatment (median exposure, 81 weeks); at the data cutoff for the week 80 anaysis, 91 (82.7%) patients in the Rux arm were still receiving treatment (median exposure, 111 weeks). No patients remained on BAT at the data cutoff for the week 80 analysis, compared with 3 patients at the week 48 analysis. The primary endpoint was achieved in 23 (20.9%) patients in the Rux arm vs 1 (0.9%) patient in the BAT arm (P < 0.0001). Among the responders in the Rux arm, only 1 patient lost this response (Figure). Overall, 60.0% of Rux patients vs 19.6% of BAT patients achieved Hct control without phlebotomy through week 32; patients achieving Hct control in the Rux arm had an 89% probability of maintaining this response for 80 weeks from the time of initial response. Of the 98 patients on Rux at week 32, 89.8% did not have a phlebotomy between weeks 32 and 80. At week 32, 38.2% vs 0.9% of patients in the Rux vs BAT arm achieved a ≥35% reduction in spleen volume; all Rux patients maintained their response. At week 32, CHR was achieved in 23.6% of Rux patients and 8.9% of BAT patients, with Rux responders having a 69% probability of maintaining CHR for 80 weeks. Among patients who discontinued, 5 of 10 in the Rux arm with available data for the Pruritus Symptom Impact Scale at their end-of-study visit rated their pruritus as “very much improved.” Nonhematologic adverse events were mainly grade 1 or 2; the most common in the Rux arm included headache (21.8% at the week 80 analysis [ie, entire follow-up] vs 20.9% at the week 48 analysis), diarrhea (20.0% vs 19.1%), pruritus (20.0% vs 17.3%), and fatigue (17.3% vs 17.3%). Grade 3 or 4 anemia and thrombocytopenia in the Rux arm did not increase from the week 48 analysis, occurring in 1.8% and 5.5% of patients, respectively. Treatment discontinuation due to adverse events remained low in the Rux arm (4.5%).
Summary
In the RESPONSE study, Rux benefit was durable and treatment remained generally well tolerated, with 82.7% still receiving Rux at a median exposure of 111 weeks.
Keyword(s): Hydroxyurea, Kinase inhibitor, Myeloproliferative disorder, Polycythemia vera
Session topic: MPN: Prognosis and treatment
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A8
Background
RESPONSE, an ongoing, multicenter, open-label, phase 3 trial, compared the efficacy and safety of ruxolitinib (Rux) with best available therapy (BAT) in patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Primary analysis results — at 48 weeks from last patient first treatment (LPFT) — have been published (Vannucchi. NEJM 2015;372(5):426-435).
Aims
To perform a second preplanned analysis assessing the long-term efficacy and safety of Rux 80 weeks after LPFT.
Methods
Patients ≥18 years of age, who were resistant to or intolerant of hydroxyurea per modified ELN criteria, with splenomegaly, and phlebotomy requirement to control hematocrit (Hct) were eligible. Patients were randomized 1:1 to receive open-label Rux 10 mg BID or BAT; the latter was selected based on investigator’s choice. Patients randomized to BAT could cross over to Rux from week 32. The primary response was a composite of (1) achieving a ≥35% reduction from baseline in spleen volume by imaging at week 32 and (2) Hct control without phlebotomy through week 32 (defined as no phlebotomy eligibility between weeks 8 to 32 with no more than 1 phlebotomy eligibility from randomization to week 8). Durability of the primary response, Hct control, spleen volume reduction, complete hematologic remission (CHR), as well as long-term safety, were evaluated.
Results
Overall, 222 patients were randomized (Rux, 110; BAT, 112). At the week 48 analysis, 93 (84.5%) patients randomized to Rux (Rux arm) were still receiving treatment (median exposure, 81 weeks); at the data cutoff for the week 80 anaysis, 91 (82.7%) patients in the Rux arm were still receiving treatment (median exposure, 111 weeks). No patients remained on BAT at the data cutoff for the week 80 analysis, compared with 3 patients at the week 48 analysis. The primary endpoint was achieved in 23 (20.9%) patients in the Rux arm vs 1 (0.9%) patient in the BAT arm (P < 0.0001). Among the responders in the Rux arm, only 1 patient lost this response (Figure). Overall, 60.0% of Rux patients vs 19.6% of BAT patients achieved Hct control without phlebotomy through week 32; patients achieving Hct control in the Rux arm had an 89% probability of maintaining this response for 80 weeks from the time of initial response. Of the 98 patients on Rux at week 32, 89.8% did not have a phlebotomy between weeks 32 and 80. At week 32, 38.2% vs 0.9% of patients in the Rux vs BAT arm achieved a ≥35% reduction in spleen volume; all Rux patients maintained their response. At week 32, CHR was achieved in 23.6% of Rux patients and 8.9% of BAT patients, with Rux responders having a 69% probability of maintaining CHR for 80 weeks. Among patients who discontinued, 5 of 10 in the Rux arm with available data for the Pruritus Symptom Impact Scale at their end-of-study visit rated their pruritus as “very much improved.” Nonhematologic adverse events were mainly grade 1 or 2; the most common in the Rux arm included headache (21.8% at the week 80 analysis [ie, entire follow-up] vs 20.9% at the week 48 analysis), diarrhea (20.0% vs 19.1%), pruritus (20.0% vs 17.3%), and fatigue (17.3% vs 17.3%). Grade 3 or 4 anemia and thrombocytopenia in the Rux arm did not increase from the week 48 analysis, occurring in 1.8% and 5.5% of patients, respectively. Treatment discontinuation due to adverse events remained low in the Rux arm (4.5%).
Summary
In the RESPONSE study, Rux benefit was durable and treatment remained generally well tolerated, with 82.7% still receiving Rux at a median exposure of 111 weeks.
Keyword(s): Hydroxyurea, Kinase inhibitor, Myeloproliferative disorder, Polycythemia vera

Session topic: MPN: Prognosis and treatment
Abstract: S447
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A8
Background
RESPONSE, an ongoing, multicenter, open-label, phase 3 trial, compared the efficacy and safety of ruxolitinib (Rux) with best available therapy (BAT) in patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Primary analysis results — at 48 weeks from last patient first treatment (LPFT) — have been published (Vannucchi. NEJM 2015;372(5):426-435).
Aims
To perform a second preplanned analysis assessing the long-term efficacy and safety of Rux 80 weeks after LPFT.
Methods
Patients ≥18 years of age, who were resistant to or intolerant of hydroxyurea per modified ELN criteria, with splenomegaly, and phlebotomy requirement to control hematocrit (Hct) were eligible. Patients were randomized 1:1 to receive open-label Rux 10 mg BID or BAT; the latter was selected based on investigator’s choice. Patients randomized to BAT could cross over to Rux from week 32. The primary response was a composite of (1) achieving a ≥35% reduction from baseline in spleen volume by imaging at week 32 and (2) Hct control without phlebotomy through week 32 (defined as no phlebotomy eligibility between weeks 8 to 32 with no more than 1 phlebotomy eligibility from randomization to week 8). Durability of the primary response, Hct control, spleen volume reduction, complete hematologic remission (CHR), as well as long-term safety, were evaluated.
Results
Overall, 222 patients were randomized (Rux, 110; BAT, 112). At the week 48 analysis, 93 (84.5%) patients randomized to Rux (Rux arm) were still receiving treatment (median exposure, 81 weeks); at the data cutoff for the week 80 anaysis, 91 (82.7%) patients in the Rux arm were still receiving treatment (median exposure, 111 weeks). No patients remained on BAT at the data cutoff for the week 80 analysis, compared with 3 patients at the week 48 analysis. The primary endpoint was achieved in 23 (20.9%) patients in the Rux arm vs 1 (0.9%) patient in the BAT arm (P < 0.0001). Among the responders in the Rux arm, only 1 patient lost this response (Figure). Overall, 60.0% of Rux patients vs 19.6% of BAT patients achieved Hct control without phlebotomy through week 32; patients achieving Hct control in the Rux arm had an 89% probability of maintaining this response for 80 weeks from the time of initial response. Of the 98 patients on Rux at week 32, 89.8% did not have a phlebotomy between weeks 32 and 80. At week 32, 38.2% vs 0.9% of patients in the Rux vs BAT arm achieved a ≥35% reduction in spleen volume; all Rux patients maintained their response. At week 32, CHR was achieved in 23.6% of Rux patients and 8.9% of BAT patients, with Rux responders having a 69% probability of maintaining CHR for 80 weeks. Among patients who discontinued, 5 of 10 in the Rux arm with available data for the Pruritus Symptom Impact Scale at their end-of-study visit rated their pruritus as “very much improved.” Nonhematologic adverse events were mainly grade 1 or 2; the most common in the Rux arm included headache (21.8% at the week 80 analysis [ie, entire follow-up] vs 20.9% at the week 48 analysis), diarrhea (20.0% vs 19.1%), pruritus (20.0% vs 17.3%), and fatigue (17.3% vs 17.3%). Grade 3 or 4 anemia and thrombocytopenia in the Rux arm did not increase from the week 48 analysis, occurring in 1.8% and 5.5% of patients, respectively. Treatment discontinuation due to adverse events remained low in the Rux arm (4.5%).
Summary
In the RESPONSE study, Rux benefit was durable and treatment remained generally well tolerated, with 82.7% still receiving Rux at a median exposure of 111 weeks.
Keyword(s): Hydroxyurea, Kinase inhibitor, Myeloproliferative disorder, Polycythemia vera

Session topic: MPN: Prognosis and treatment
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 11:45 to 13.06.2015 12:00
Location: Room A8
Background
RESPONSE, an ongoing, multicenter, open-label, phase 3 trial, compared the efficacy and safety of ruxolitinib (Rux) with best available therapy (BAT) in patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. Primary analysis results — at 48 weeks from last patient first treatment (LPFT) — have been published (Vannucchi. NEJM 2015;372(5):426-435).
Aims
To perform a second preplanned analysis assessing the long-term efficacy and safety of Rux 80 weeks after LPFT.
Methods
Patients ≥18 years of age, who were resistant to or intolerant of hydroxyurea per modified ELN criteria, with splenomegaly, and phlebotomy requirement to control hematocrit (Hct) were eligible. Patients were randomized 1:1 to receive open-label Rux 10 mg BID or BAT; the latter was selected based on investigator’s choice. Patients randomized to BAT could cross over to Rux from week 32. The primary response was a composite of (1) achieving a ≥35% reduction from baseline in spleen volume by imaging at week 32 and (2) Hct control without phlebotomy through week 32 (defined as no phlebotomy eligibility between weeks 8 to 32 with no more than 1 phlebotomy eligibility from randomization to week 8). Durability of the primary response, Hct control, spleen volume reduction, complete hematologic remission (CHR), as well as long-term safety, were evaluated.
Results
Overall, 222 patients were randomized (Rux, 110; BAT, 112). At the week 48 analysis, 93 (84.5%) patients randomized to Rux (Rux arm) were still receiving treatment (median exposure, 81 weeks); at the data cutoff for the week 80 anaysis, 91 (82.7%) patients in the Rux arm were still receiving treatment (median exposure, 111 weeks). No patients remained on BAT at the data cutoff for the week 80 analysis, compared with 3 patients at the week 48 analysis. The primary endpoint was achieved in 23 (20.9%) patients in the Rux arm vs 1 (0.9%) patient in the BAT arm (P < 0.0001). Among the responders in the Rux arm, only 1 patient lost this response (Figure). Overall, 60.0% of Rux patients vs 19.6% of BAT patients achieved Hct control without phlebotomy through week 32; patients achieving Hct control in the Rux arm had an 89% probability of maintaining this response for 80 weeks from the time of initial response. Of the 98 patients on Rux at week 32, 89.8% did not have a phlebotomy between weeks 32 and 80. At week 32, 38.2% vs 0.9% of patients in the Rux vs BAT arm achieved a ≥35% reduction in spleen volume; all Rux patients maintained their response. At week 32, CHR was achieved in 23.6% of Rux patients and 8.9% of BAT patients, with Rux responders having a 69% probability of maintaining CHR for 80 weeks. Among patients who discontinued, 5 of 10 in the Rux arm with available data for the Pruritus Symptom Impact Scale at their end-of-study visit rated their pruritus as “very much improved.” Nonhematologic adverse events were mainly grade 1 or 2; the most common in the Rux arm included headache (21.8% at the week 80 analysis [ie, entire follow-up] vs 20.9% at the week 48 analysis), diarrhea (20.0% vs 19.1%), pruritus (20.0% vs 17.3%), and fatigue (17.3% vs 17.3%). Grade 3 or 4 anemia and thrombocytopenia in the Rux arm did not increase from the week 48 analysis, occurring in 1.8% and 5.5% of patients, respectively. Treatment discontinuation due to adverse events remained low in the Rux arm (4.5%).
Summary
In the RESPONSE study, Rux benefit was durable and treatment remained generally well tolerated, with 82.7% still receiving Rux at a median exposure of 111 weeks.
Keyword(s): Hydroxyurea, Kinase inhibitor, Myeloproliferative disorder, Polycythemia vera

Session topic: MPN: Prognosis and treatment
{{ help_message }}
{{filter}}


