A RANDOMIZED, OPEN-LABEL, PHASE 2 STUDY OF BORTEZOMIB AND DEXAMETHASONE WITH OR WITHOUT ELOTUZUMAB IN PATIENTS WITH RELAPSED/REFRACTORY MULTIPLE MYELOMA
(Abstract release date: 05/21/15)
EHA Library. J. Jakubowiak A. 06/12/15; 103091; S103

Andrzej J. Jakubowiak
Contributions
Contributions
Abstract
Abstract: S103
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room A2+3
Background
Elotuzumab, an immunotherapeutic monoclonal antibody (mAb), recognizes Signaling Lymphocytic Activation Molecule F7 (SLAMF7), a protein highly expressed on myeloma cells and natural killer cells. Elotuzumab targets and kills SLAMF7-expressing myeloma cells by direct activation of natural killer cells with minimal effect on normal tissues. Elotuzumab demonstrated enhanced activity when combined with bortezomib in a preclinical myeloma model,1 and elotuzumab/bortezomib showed encouraging clinical activity in a Phase 1 study.2
Aims
This Phase 2 open-label study (NCT01478048, CA204-009) aimed to investigate the efficacy and safety of elotuzumab + bortezomib/dexamethasone (Bd) compared with Bd alone in patients (pts) with relapsed/refractory multiple myeloma (RRMM).
Methods
Pts with RRMM who had received 1–3 prior therapies were given elotuzumab + Bd (EBd) or Bd in 21-day (Cycles 1–8) or 28-day Cycles (9+) until disease progression or unacceptable toxicity. Dosing schedule: elotuzumab (10 mg/kg IV) weekly Cycles 1–2, Day 1 and 11 Cycles 3–8, then Day 1 and 15; bortezomib (1.3 mg/m2 IV/SC) Day 1, 4, 8, and 11 Cycles 1–8, then Day 1, 8, and 15; dexamethasone 20 mg on non-elotuzumab days, 8 mg PO + 8 mg IV on elotuzumab days. The primary endpoint was progression-free survival (PFS; ITT population) according to IMWG criteria. In this proof-of-concept study, a 2-sided 0.30 significance level was specified to test for PFS difference between arms; p≤0.3 was considered significant. The study had 80% power to detect a hazard ratio (HR) of 0.69 with 103 events. Informed written consent was obtained for all participants.
Results
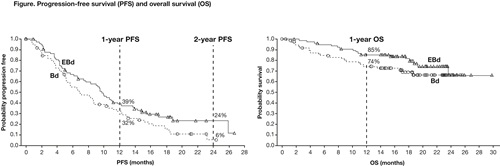
In total, 152 pts (median age 66 years) were randomized to either EBd (77) or Bd (75). At data cut-off (12 Sep 2014), 18% of pts treated with EBd vs 10% of pts treated with Bd remained on therapy. The median number of treatment cycles was 12 with EBd and 7 with Bd. Discontinuation was mainly for disease progression (52%). HR for PFS was 0.71 (70% CI 0.58, 0.87; p=0.08). One-year PFS rate was 39% (95% CI 28%, 50%) in the EBd group vs 32% (95% CI 21%, 44%) in the Bd group, and 2-year PFS rate was 24% (95% CI 13%, 36%) in the EBd group and 6% (95% CI 1%, 19%) in the Bd group (Figure). PFS HR (EBd vs Bd), adjusting for prognostic factors, was 0.58 (70% CI 0.47, 0.72; p=0.01). The median PFS observed with EBd was 9.7 months, vs 6.9 months with Bd. Overall response rate was 66% with EBd vs 63% with Bd. Early overall survival (OS) results revealed a HR of 0.61 (70% CI 0.43, 0.85); 1-year OS rate was 85% (95% CI 75%, 92%) in the EBd group vs 74% (95% CI 62%, 83%) in the Bd group (Figure). Forty deaths (17 EBd, 23 Bd) were observed at the time of analysis, mainly due to disease progression. Follow-up for OS continues. Grade 3/4 adverse events (AEs) were reported in 51 (68%) pts with EBd treatment vs 45 (60%) with Bd. AEs ≥Grade 3 that occurred in ≥15% of pts in each group were thrombocytopenia (7 [9%] in EBd group; 13 [17%] in Bd group) and infections (14 [19%] in EBd group; 11 [15%] in Bd group). Infusion reactions (IRs; all Grade 1–2) occurred in 7% of pts treated with EBd. There were no IRs at the maximum planned 5 mL/min infusion rate.
Summary
This study met the primary endpoint: PFS was longer with EBd than with Bd. More pts continued on EBd than Bd and early OS data favor EBd. Rate of IRs was low with EBd, and IRs were manageable with premedication. In pts with RRMM, elotuzumab, an immunotherapeutic mAb, provides clinical benefit with limited added toxicity when combined with Bd vs Bd alone.
Keyword(s): Bortezomib, Monoclonal antibody, Multiple myeloma, Survival
Session topic: Multiple myeloma: Clinical studies 1
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room A2+3
Background
Elotuzumab, an immunotherapeutic monoclonal antibody (mAb), recognizes Signaling Lymphocytic Activation Molecule F7 (SLAMF7), a protein highly expressed on myeloma cells and natural killer cells. Elotuzumab targets and kills SLAMF7-expressing myeloma cells by direct activation of natural killer cells with minimal effect on normal tissues. Elotuzumab demonstrated enhanced activity when combined with bortezomib in a preclinical myeloma model,1 and elotuzumab/bortezomib showed encouraging clinical activity in a Phase 1 study.2
Aims
This Phase 2 open-label study (NCT01478048, CA204-009) aimed to investigate the efficacy and safety of elotuzumab + bortezomib/dexamethasone (Bd) compared with Bd alone in patients (pts) with relapsed/refractory multiple myeloma (RRMM).
Methods
Pts with RRMM who had received 1–3 prior therapies were given elotuzumab + Bd (EBd) or Bd in 21-day (Cycles 1–8) or 28-day Cycles (9+) until disease progression or unacceptable toxicity. Dosing schedule: elotuzumab (10 mg/kg IV) weekly Cycles 1–2, Day 1 and 11 Cycles 3–8, then Day 1 and 15; bortezomib (1.3 mg/m2 IV/SC) Day 1, 4, 8, and 11 Cycles 1–8, then Day 1, 8, and 15; dexamethasone 20 mg on non-elotuzumab days, 8 mg PO + 8 mg IV on elotuzumab days. The primary endpoint was progression-free survival (PFS; ITT population) according to IMWG criteria. In this proof-of-concept study, a 2-sided 0.30 significance level was specified to test for PFS difference between arms; p≤0.3 was considered significant. The study had 80% power to detect a hazard ratio (HR) of 0.69 with 103 events. Informed written consent was obtained for all participants.
Results
In total, 152 pts (median age 66 years) were randomized to either EBd (77) or Bd (75). At data cut-off (12 Sep 2014), 18% of pts treated with EBd vs 10% of pts treated with Bd remained on therapy. The median number of treatment cycles was 12 with EBd and 7 with Bd. Discontinuation was mainly for disease progression (52%). HR for PFS was 0.71 (70% CI 0.58, 0.87; p=0.08). One-year PFS rate was 39% (95% CI 28%, 50%) in the EBd group vs 32% (95% CI 21%, 44%) in the Bd group, and 2-year PFS rate was 24% (95% CI 13%, 36%) in the EBd group and 6% (95% CI 1%, 19%) in the Bd group (Figure). PFS HR (EBd vs Bd), adjusting for prognostic factors, was 0.58 (70% CI 0.47, 0.72; p=0.01). The median PFS observed with EBd was 9.7 months, vs 6.9 months with Bd. Overall response rate was 66% with EBd vs 63% with Bd. Early overall survival (OS) results revealed a HR of 0.61 (70% CI 0.43, 0.85); 1-year OS rate was 85% (95% CI 75%, 92%) in the EBd group vs 74% (95% CI 62%, 83%) in the Bd group (Figure). Forty deaths (17 EBd, 23 Bd) were observed at the time of analysis, mainly due to disease progression. Follow-up for OS continues. Grade 3/4 adverse events (AEs) were reported in 51 (68%) pts with EBd treatment vs 45 (60%) with Bd. AEs ≥Grade 3 that occurred in ≥15% of pts in each group were thrombocytopenia (7 [9%] in EBd group; 13 [17%] in Bd group) and infections (14 [19%] in EBd group; 11 [15%] in Bd group). Infusion reactions (IRs; all Grade 1–2) occurred in 7% of pts treated with EBd. There were no IRs at the maximum planned 5 mL/min infusion rate.
Summary
This study met the primary endpoint: PFS was longer with EBd than with Bd. More pts continued on EBd than Bd and early OS data favor EBd. Rate of IRs was low with EBd, and IRs were manageable with premedication. In pts with RRMM, elotuzumab, an immunotherapeutic mAb, provides clinical benefit with limited added toxicity when combined with Bd vs Bd alone.
References: 1. van Rhee F et al. Mol Cancer Ther 2009;8:2616–24. 2. Jakubowiak A et al. J Clin Oncol 2012;30:1960–65.
Keyword(s): Bortezomib, Monoclonal antibody, Multiple myeloma, Survival

Session topic: Multiple myeloma: Clinical studies 1
Abstract: S103
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room A2+3
Background
Elotuzumab, an immunotherapeutic monoclonal antibody (mAb), recognizes Signaling Lymphocytic Activation Molecule F7 (SLAMF7), a protein highly expressed on myeloma cells and natural killer cells. Elotuzumab targets and kills SLAMF7-expressing myeloma cells by direct activation of natural killer cells with minimal effect on normal tissues. Elotuzumab demonstrated enhanced activity when combined with bortezomib in a preclinical myeloma model,1 and elotuzumab/bortezomib showed encouraging clinical activity in a Phase 1 study.2
Aims
This Phase 2 open-label study (NCT01478048, CA204-009) aimed to investigate the efficacy and safety of elotuzumab + bortezomib/dexamethasone (Bd) compared with Bd alone in patients (pts) with relapsed/refractory multiple myeloma (RRMM).
Methods
Pts with RRMM who had received 1–3 prior therapies were given elotuzumab + Bd (EBd) or Bd in 21-day (Cycles 1–8) or 28-day Cycles (9+) until disease progression or unacceptable toxicity. Dosing schedule: elotuzumab (10 mg/kg IV) weekly Cycles 1–2, Day 1 and 11 Cycles 3–8, then Day 1 and 15; bortezomib (1.3 mg/m2 IV/SC) Day 1, 4, 8, and 11 Cycles 1–8, then Day 1, 8, and 15; dexamethasone 20 mg on non-elotuzumab days, 8 mg PO + 8 mg IV on elotuzumab days. The primary endpoint was progression-free survival (PFS; ITT population) according to IMWG criteria. In this proof-of-concept study, a 2-sided 0.30 significance level was specified to test for PFS difference between arms; p≤0.3 was considered significant. The study had 80% power to detect a hazard ratio (HR) of 0.69 with 103 events. Informed written consent was obtained for all participants.
Results
In total, 152 pts (median age 66 years) were randomized to either EBd (77) or Bd (75). At data cut-off (12 Sep 2014), 18% of pts treated with EBd vs 10% of pts treated with Bd remained on therapy. The median number of treatment cycles was 12 with EBd and 7 with Bd. Discontinuation was mainly for disease progression (52%). HR for PFS was 0.71 (70% CI 0.58, 0.87; p=0.08). One-year PFS rate was 39% (95% CI 28%, 50%) in the EBd group vs 32% (95% CI 21%, 44%) in the Bd group, and 2-year PFS rate was 24% (95% CI 13%, 36%) in the EBd group and 6% (95% CI 1%, 19%) in the Bd group (Figure). PFS HR (EBd vs Bd), adjusting for prognostic factors, was 0.58 (70% CI 0.47, 0.72; p=0.01). The median PFS observed with EBd was 9.7 months, vs 6.9 months with Bd. Overall response rate was 66% with EBd vs 63% with Bd. Early overall survival (OS) results revealed a HR of 0.61 (70% CI 0.43, 0.85); 1-year OS rate was 85% (95% CI 75%, 92%) in the EBd group vs 74% (95% CI 62%, 83%) in the Bd group (Figure). Forty deaths (17 EBd, 23 Bd) were observed at the time of analysis, mainly due to disease progression. Follow-up for OS continues. Grade 3/4 adverse events (AEs) were reported in 51 (68%) pts with EBd treatment vs 45 (60%) with Bd. AEs ≥Grade 3 that occurred in ≥15% of pts in each group were thrombocytopenia (7 [9%] in EBd group; 13 [17%] in Bd group) and infections (14 [19%] in EBd group; 11 [15%] in Bd group). Infusion reactions (IRs; all Grade 1–2) occurred in 7% of pts treated with EBd. There were no IRs at the maximum planned 5 mL/min infusion rate.
Summary
This study met the primary endpoint: PFS was longer with EBd than with Bd. More pts continued on EBd than Bd and early OS data favor EBd. Rate of IRs was low with EBd, and IRs were manageable with premedication. In pts with RRMM, elotuzumab, an immunotherapeutic mAb, provides clinical benefit with limited added toxicity when combined with Bd vs Bd alone.
Keyword(s): Bortezomib, Monoclonal antibody, Multiple myeloma, Survival

Session topic: Multiple myeloma: Clinical studies 1
Type: Oral Presentation
Presentation during EHA20: From 12.06.2015 12:00 to 12.06.2015 12:15
Location: Room A2+3
Background
Elotuzumab, an immunotherapeutic monoclonal antibody (mAb), recognizes Signaling Lymphocytic Activation Molecule F7 (SLAMF7), a protein highly expressed on myeloma cells and natural killer cells. Elotuzumab targets and kills SLAMF7-expressing myeloma cells by direct activation of natural killer cells with minimal effect on normal tissues. Elotuzumab demonstrated enhanced activity when combined with bortezomib in a preclinical myeloma model,1 and elotuzumab/bortezomib showed encouraging clinical activity in a Phase 1 study.2
Aims
This Phase 2 open-label study (NCT01478048, CA204-009) aimed to investigate the efficacy and safety of elotuzumab + bortezomib/dexamethasone (Bd) compared with Bd alone in patients (pts) with relapsed/refractory multiple myeloma (RRMM).
Methods
Pts with RRMM who had received 1–3 prior therapies were given elotuzumab + Bd (EBd) or Bd in 21-day (Cycles 1–8) or 28-day Cycles (9+) until disease progression or unacceptable toxicity. Dosing schedule: elotuzumab (10 mg/kg IV) weekly Cycles 1–2, Day 1 and 11 Cycles 3–8, then Day 1 and 15; bortezomib (1.3 mg/m2 IV/SC) Day 1, 4, 8, and 11 Cycles 1–8, then Day 1, 8, and 15; dexamethasone 20 mg on non-elotuzumab days, 8 mg PO + 8 mg IV on elotuzumab days. The primary endpoint was progression-free survival (PFS; ITT population) according to IMWG criteria. In this proof-of-concept study, a 2-sided 0.30 significance level was specified to test for PFS difference between arms; p≤0.3 was considered significant. The study had 80% power to detect a hazard ratio (HR) of 0.69 with 103 events. Informed written consent was obtained for all participants.
Results
In total, 152 pts (median age 66 years) were randomized to either EBd (77) or Bd (75). At data cut-off (12 Sep 2014), 18% of pts treated with EBd vs 10% of pts treated with Bd remained on therapy. The median number of treatment cycles was 12 with EBd and 7 with Bd. Discontinuation was mainly for disease progression (52%). HR for PFS was 0.71 (70% CI 0.58, 0.87; p=0.08). One-year PFS rate was 39% (95% CI 28%, 50%) in the EBd group vs 32% (95% CI 21%, 44%) in the Bd group, and 2-year PFS rate was 24% (95% CI 13%, 36%) in the EBd group and 6% (95% CI 1%, 19%) in the Bd group (Figure). PFS HR (EBd vs Bd), adjusting for prognostic factors, was 0.58 (70% CI 0.47, 0.72; p=0.01). The median PFS observed with EBd was 9.7 months, vs 6.9 months with Bd. Overall response rate was 66% with EBd vs 63% with Bd. Early overall survival (OS) results revealed a HR of 0.61 (70% CI 0.43, 0.85); 1-year OS rate was 85% (95% CI 75%, 92%) in the EBd group vs 74% (95% CI 62%, 83%) in the Bd group (Figure). Forty deaths (17 EBd, 23 Bd) were observed at the time of analysis, mainly due to disease progression. Follow-up for OS continues. Grade 3/4 adverse events (AEs) were reported in 51 (68%) pts with EBd treatment vs 45 (60%) with Bd. AEs ≥Grade 3 that occurred in ≥15% of pts in each group were thrombocytopenia (7 [9%] in EBd group; 13 [17%] in Bd group) and infections (14 [19%] in EBd group; 11 [15%] in Bd group). Infusion reactions (IRs; all Grade 1–2) occurred in 7% of pts treated with EBd. There were no IRs at the maximum planned 5 mL/min infusion rate.
Summary
This study met the primary endpoint: PFS was longer with EBd than with Bd. More pts continued on EBd than Bd and early OS data favor EBd. Rate of IRs was low with EBd, and IRs were manageable with premedication. In pts with RRMM, elotuzumab, an immunotherapeutic mAb, provides clinical benefit with limited added toxicity when combined with Bd vs Bd alone.
References: 1. van Rhee F et al. Mol Cancer Ther 2009;8:2616–24. 2. Jakubowiak A et al. J Clin Oncol 2012;30:1960–65.
Keyword(s): Bortezomib, Monoclonal antibody, Multiple myeloma, Survival

Session topic: Multiple myeloma: Clinical studies 1
{{ help_message }}
{{filter}}


