
Contributions
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:30 to 13.06.2015 12:45
Location: Room A8
Background
JAK1/2 inhibitors (JAK#) have the potential to effectively control MF-related symptoms, and improve the clinical status prior to HCT. However, the clinical experience is limited, and data are conflicting. (Robin et al, Blood, 2013, ASH abstract 306; Stübig et al, Leukemia, 2014). Additionally, the relationship between the response to JAK #, and outcomes of HCT is not clear.
Aims
To report outcomes of HCT in MF patients exposed to JAK#
Methods
In this multi-institutional retrospective study, we evaluated the outcomes of 93 MF patients (pts.) who had exposure to JAK# prior to HCT. A working definition of response to JAK# was established inspired by International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) criteria. (Table1). The primary end point was overall survival (OS) from the date of HCT.
|
Results
Median age at HCT was 59 years (range 32-72 years). Diagnosis was primary-MF (n=53), post polycythemia-MF (n=20), or post essential thrombocythemia-MF (n=20). DIPSS-plus score at the time of HCT was low in 2 (2%), INT-1 in 19 (22%), INT-2 in 47 (52%), high-risk in 22 (24%), and not available in 3 pts. Conditioning was full intensity in 42 (45%), and reduced intensity in 51 (55%). Donors were matched sibling in 36 (39%), matched unrelated in 47 (51%), and mismatched or haplo-identical in 10 (11%). JAK# used were ruxolitinib (n=84), momelitinib (n=6), or others (n=3). Sixty-five (70%) pts used JAK# leading to HCT, and stopped 0-16 days prior to conditioning regimen; 23 (25%) pts had discontinued the medication at least 4 weeks prior to HCT due to progression or intolerance, and in 5 pts this information was not available.
Among pts who used JAK# leading to HCT, “withdrawal symptoms” were reported in 10 (15%), and were more common in pts who stopped JAK inhibitors ≥ 6 days prior to conditioning regimen compared to those who stopped within 0-6 days (26% vs. 11%, p=0.06). Withdrawal symptoms were non-severe in nature except in one pt. this resulted in rebound splenomegaly and pulmonary infiltrates necessitating splenectomy and delaying the HCT for 3 months. Primary graft failure was reported in 3 pts (3%). The cumulative incidence of grade ≥2, and ≥3 acute GVHD at 100 days were 44% and 16% respectively. Cumulative incidence of chronic GVHD at 2-years was 53%.
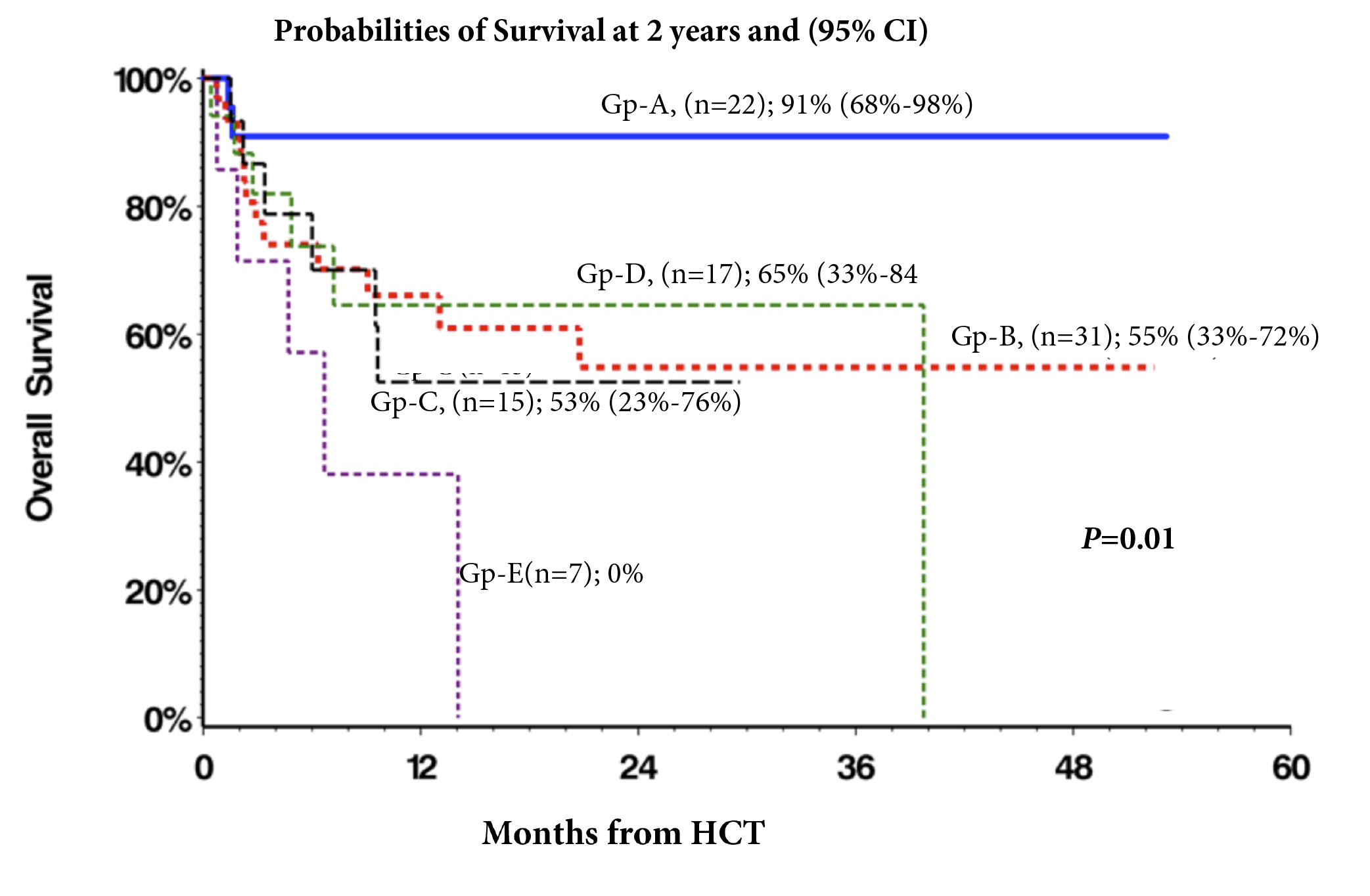
Median follow-up of survivors were (2-53) months, and during this period, 12 (13%) pts had relapse/progression and 31 (33%) died. Probability of 2-year OS of whole cohort was 62% (95% CI, 50%>73%). Patient in group-A (n=22) had significantly superior survival p=0.01(Fig.1) Other factors with significant effect on survival in univariate analysis were DIPSS-plus score, donor type, and performance status at HCT. In a limited multivariate analysis, response to JAK# was the strongest independent factor for survival (p=0.004). DIPSS-plus high-risk category (p=0.04), and mismatched/haplo donor groups (p=0.04) were other independent factors for survival.
Summary
Our data suggest that use of JAK# prior to HCT does not have adverse effects on early transplant outcomes. Continuation of JAK# until HCT appears to reduce the risk of “withdrawal symptoms”. The HCT outcomes in patients responding to JAK# are particularly encouraging.
Keyword(s): Myelofibrosis, Transplant
Session topic: MPN: Prognosis and treatment
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:30 to 13.06.2015 12:45
Location: Room A8
Background
JAK1/2 inhibitors (JAK#) have the potential to effectively control MF-related symptoms, and improve the clinical status prior to HCT. However, the clinical experience is limited, and data are conflicting. (Robin et al, Blood, 2013, ASH abstract 306; Stübig et al, Leukemia, 2014). Additionally, the relationship between the response to JAK #, and outcomes of HCT is not clear.
Aims
To report outcomes of HCT in MF patients exposed to JAK#
Methods
In this multi-institutional retrospective study, we evaluated the outcomes of 93 MF patients (pts.) who had exposure to JAK# prior to HCT. A working definition of response to JAK# was established inspired by International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) criteria. (Table1). The primary end point was overall survival (OS) from the date of HCT.
|
Results
Median age at HCT was 59 years (range 32-72 years). Diagnosis was primary-MF (n=53), post polycythemia-MF (n=20), or post essential thrombocythemia-MF (n=20). DIPSS-plus score at the time of HCT was low in 2 (2%), INT-1 in 19 (22%), INT-2 in 47 (52%), high-risk in 22 (24%), and not available in 3 pts. Conditioning was full intensity in 42 (45%), and reduced intensity in 51 (55%). Donors were matched sibling in 36 (39%), matched unrelated in 47 (51%), and mismatched or haplo-identical in 10 (11%). JAK# used were ruxolitinib (n=84), momelitinib (n=6), or others (n=3). Sixty-five (70%) pts used JAK# leading to HCT, and stopped 0-16 days prior to conditioning regimen; 23 (25%) pts had discontinued the medication at least 4 weeks prior to HCT due to progression or intolerance, and in 5 pts this information was not available.
Among pts who used JAK# leading to HCT, “withdrawal symptoms” were reported in 10 (15%), and were more common in pts who stopped JAK inhibitors ≥ 6 days prior to conditioning regimen compared to those who stopped within 0-6 days (26% vs. 11%, p=0.06). Withdrawal symptoms were non-severe in nature except in one pt. this resulted in rebound splenomegaly and pulmonary infiltrates necessitating splenectomy and delaying the HCT for 3 months. Primary graft failure was reported in 3 pts (3%). The cumulative incidence of grade ≥2, and ≥3 acute GVHD at 100 days were 44% and 16% respectively. Cumulative incidence of chronic GVHD at 2-years was 53%.
Median follow-up of survivors were (2-53) months, and during this period, 12 (13%) pts had relapse/progression and 31 (33%) died. Probability of 2-year OS of whole cohort was 62% (95% CI, 50%>73%). Patient in group-A (n=22) had significantly superior survival p=0.01(Fig.1) Other factors with significant effect on survival in univariate analysis were DIPSS-plus score, donor type, and performance status at HCT. In a limited multivariate analysis, response to JAK# was the strongest independent factor for survival (p=0.004). DIPSS-plus high-risk category (p=0.04), and mismatched/haplo donor groups (p=0.04) were other independent factors for survival.
Summary
Our data suggest that use of JAK# prior to HCT does not have adverse effects on early transplant outcomes. Continuation of JAK# until HCT appears to reduce the risk of “withdrawal symptoms”. The HCT outcomes in patients responding to JAK# are particularly encouraging.
Keyword(s): Myelofibrosis, Transplant

Session topic: MPN: Prognosis and treatment


