SAFETY OF IDELALISIB IN B-CELL MALIGNANCIES: INTEGRATED ANALYSIS OF EIGHT CLINICAL TRIALS
(Abstract release date: 05/21/15)
EHA Library. Coutre S. 06/13/15; 100729; S433

Dr. Steven E Coutre
Contributions
Contributions
Abstract
Abstract: S433
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:00 13.06.2015 12:15
Location: Room A7
Background
Idelalisib (Zydelig), a first-in-class, selective, oral inhibitor of PI3Kδ, is approved in the US and EU for the treatment of chronic lymphocytic leukemia (CLL) in combination with rituximab and as monotherapy for patients with follicular lymphoma who have received at least two prior systemic therapies.
Aims
To further characterize the safety profile of idelalisib, we integrated adverse event data from 8 clinical trials.
Methods
The analysis included 760 subjects with CLL, indolent non-Hodgkin lymphoma, or other B-cell malignancy who received IDELA alone (doses = 50 mg BID to 350 mg BID) or as part of a combination regimen (IDELA doses = 100 or 150 mg BID). Most subjects were heavily pre-treated with relapsed disease.
Results
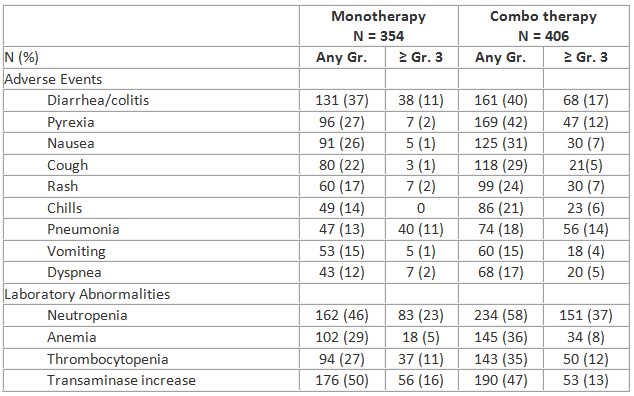
Common adverse events (AEs) are presented in the table, along with important laboratory results. AEs leading to dose modification included transaminase elevations (13%), diarrhea/colitis (11%), and rash (5%); discontinuations due to these AEs were infrequent (3%, 5%, and 2%, respectively); dose interruption allowed successful re-challenge in most patients. Pneumonitis occurred in 2.3% (monotherapy) and 3.9% (combo therapy) of subjects. Grade ≥ 3 diarrhea/colitis had a later onset (peak incidence of 9% between 6-12 mo.); other AEs occurred most often in the first 6 months and declined thereafter.
Summary
As well-characterized in this large dataset, IDELA has an acceptable safety profile.
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Non-Hodgkin's lymphoma, PI3K
Session topic: Chronic lymphocytic leukemia - Clinical 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:00 13.06.2015 12:15
Location: Room A7
Background
Idelalisib (Zydelig), a first-in-class, selective, oral inhibitor of PI3Kδ, is approved in the US and EU for the treatment of chronic lymphocytic leukemia (CLL) in combination with rituximab and as monotherapy for patients with follicular lymphoma who have received at least two prior systemic therapies.
Aims
To further characterize the safety profile of idelalisib, we integrated adverse event data from 8 clinical trials.
Methods
The analysis included 760 subjects with CLL, indolent non-Hodgkin lymphoma, or other B-cell malignancy who received IDELA alone (doses = 50 mg BID to 350 mg BID) or as part of a combination regimen (IDELA doses = 100 or 150 mg BID). Most subjects were heavily pre-treated with relapsed disease.
Results
Common adverse events (AEs) are presented in the table, along with important laboratory results. AEs leading to dose modification included transaminase elevations (13%), diarrhea/colitis (11%), and rash (5%); discontinuations due to these AEs were infrequent (3%, 5%, and 2%, respectively); dose interruption allowed successful re-challenge in most patients. Pneumonitis occurred in 2.3% (monotherapy) and 3.9% (combo therapy) of subjects. Grade ≥ 3 diarrhea/colitis had a later onset (peak incidence of 9% between 6-12 mo.); other AEs occurred most often in the first 6 months and declined thereafter.
Summary
As well-characterized in this large dataset, IDELA has an acceptable safety profile.
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Non-Hodgkin's lymphoma, PI3K

Session topic: Chronic lymphocytic leukemia - Clinical 2
Abstract: S433
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:00 13.06.2015 12:15
Location: Room A7
Background
Idelalisib (Zydelig), a first-in-class, selective, oral inhibitor of PI3Kδ, is approved in the US and EU for the treatment of chronic lymphocytic leukemia (CLL) in combination with rituximab and as monotherapy for patients with follicular lymphoma who have received at least two prior systemic therapies.
Aims
To further characterize the safety profile of idelalisib, we integrated adverse event data from 8 clinical trials.
Methods
The analysis included 760 subjects with CLL, indolent non-Hodgkin lymphoma, or other B-cell malignancy who received IDELA alone (doses = 50 mg BID to 350 mg BID) or as part of a combination regimen (IDELA doses = 100 or 150 mg BID). Most subjects were heavily pre-treated with relapsed disease.
Results
Common adverse events (AEs) are presented in the table, along with important laboratory results. AEs leading to dose modification included transaminase elevations (13%), diarrhea/colitis (11%), and rash (5%); discontinuations due to these AEs were infrequent (3%, 5%, and 2%, respectively); dose interruption allowed successful re-challenge in most patients. Pneumonitis occurred in 2.3% (monotherapy) and 3.9% (combo therapy) of subjects. Grade ≥ 3 diarrhea/colitis had a later onset (peak incidence of 9% between 6-12 mo.); other AEs occurred most often in the first 6 months and declined thereafter.
Summary
As well-characterized in this large dataset, IDELA has an acceptable safety profile.
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Non-Hodgkin's lymphoma, PI3K

Session topic: Chronic lymphocytic leukemia - Clinical 2
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 12:00 13.06.2015 12:15
Location: Room A7
Background
Idelalisib (Zydelig), a first-in-class, selective, oral inhibitor of PI3Kδ, is approved in the US and EU for the treatment of chronic lymphocytic leukemia (CLL) in combination with rituximab and as monotherapy for patients with follicular lymphoma who have received at least two prior systemic therapies.
Aims
To further characterize the safety profile of idelalisib, we integrated adverse event data from 8 clinical trials.
Methods
The analysis included 760 subjects with CLL, indolent non-Hodgkin lymphoma, or other B-cell malignancy who received IDELA alone (doses = 50 mg BID to 350 mg BID) or as part of a combination regimen (IDELA doses = 100 or 150 mg BID). Most subjects were heavily pre-treated with relapsed disease.
Results
Common adverse events (AEs) are presented in the table, along with important laboratory results. AEs leading to dose modification included transaminase elevations (13%), diarrhea/colitis (11%), and rash (5%); discontinuations due to these AEs were infrequent (3%, 5%, and 2%, respectively); dose interruption allowed successful re-challenge in most patients. Pneumonitis occurred in 2.3% (monotherapy) and 3.9% (combo therapy) of subjects. Grade ≥ 3 diarrhea/colitis had a later onset (peak incidence of 9% between 6-12 mo.); other AEs occurred most often in the first 6 months and declined thereafter.
Summary
As well-characterized in this large dataset, IDELA has an acceptable safety profile.
Keyword(s): Chronic lymphocytic leukemia, Clinical trial, Non-Hodgkin's lymphoma, PI3K

Session topic: Chronic lymphocytic leukemia - Clinical 2
{{ help_message }}
{{filter}}


